Platform
Inhibitors of the well-supported target NEU3: We identified potent NEU3 inhibitors which mimic the sialic acid transition state in NEU3. Many are < 200 Da, and some have < 2 nM IC50’s in human NEU3 activity assays (see the Target Overview page) including ability to cause human LAP to release active TGF-beta1, ability to desialyate SAP, and ability to cause human PBMC to upregulate extracellular IL-6. Some compounds are new composition of matter.
Strong efficacy and no discernible toxicity: We tried 3 of our new NEU3 inhibitors in the mouse bleomycin model of pulmonary fibrosis. Daily dosing starting at 10 days, at 1 mg/ kg for two compounds, and 0.1 mg/kg for a third, inhibited fibrosis (two showing no significant difference from no-bleomycin control). No toxicity was observed in the mice, or below 2 mM in cell-based assays. (for more information on this and the 2 figures below, see Karhadkar, T.R., Meek, T.D., and Gomer, R.H. Inhibiting sialidase-induced TGF-β1 activation attenuates pulmonary fibrosis in mice. Journal of Pharmacology and Experimental Therapeutics, 376, 106-117 (2021).)
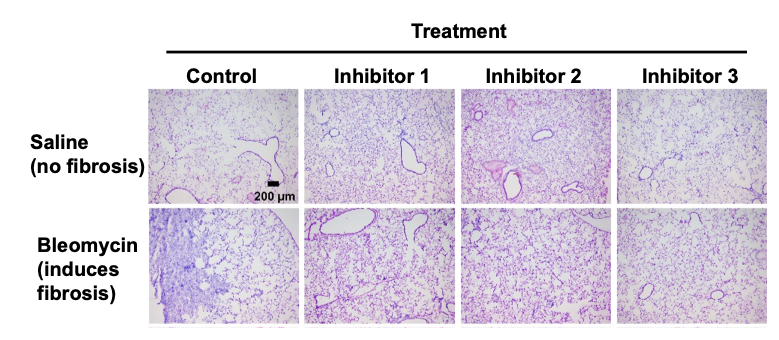
Histology indicates efficacy of inhibitors: Mice were treated with bleomycin to induce pulmonary fibrosis, or saline as a control. Starting at day 10, mice were treated daily with buffer (control), or NEU3 inhibitors. At day 21 mice were euthanized and and lung sections were stained with H&E. In the saline treated mice, the lungs show no discernible toxicity of the inhibitors. In the bleomycin-treated mouse subsequently treated with buffer, a large purple-stained fibrotic mass is visible. In the bleomycin-treated mice subsequently treated with inhibitors, there is little evidence of fibrosis.
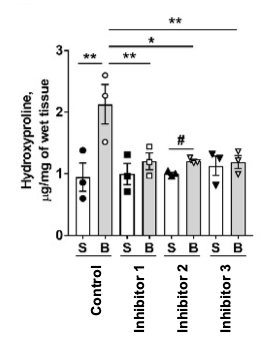
A hydroxyproline assay indicates efficacy of inhibitors: Mouse lungs from the above experiment were assayed for hydroxyproline content, a standard marker of the collagen present in fibrotic lesions. S indicates saline, B, bleomycin.
Easy surrogate marker: IPF patients and bleomycin-treated mice show elevated desialylation of serum glycoproteins, and our NEU3 inhibitors block this desialylation in the mouse bleomycin model.