Target Overview
The target is the sialidase enzyme NEU3, a key component of two out-of control positive feedback loops that drive fibrosis.
Sialic acid is often the distal sugar on glycoconjugates, and sialidase enzymes remove this sugar. The extracellular sialidase NEU3 is upregulated in fibrotic lesions in the fibrotic lesions in patients with idiopathic pulmonary fibrosis, and and the fibrotic lesions in other fibrosing diseases. The extracellular signal TGF-beta1 is a key driver of fibrosis. NEU3 potentiates fibrosis by desialylating the latent TGF-beta1 binding protein that sequesters TGF-beta1 in an inactive state, causing it to release active TGF-beta1. NEU3 also desialylates and inactivates the antifibrotic protein SAP (which Roche is using in Phase 3 trials for IPF), and causes cells to upregulate extracellular levels of the profibrotic signal IL-6. In positive feedback loops, TGF-beta1 and IL-6 upregulate NEU3 by increasing translation of existing NEU3 mRNA rather than increasing NEU3 mRNA production.
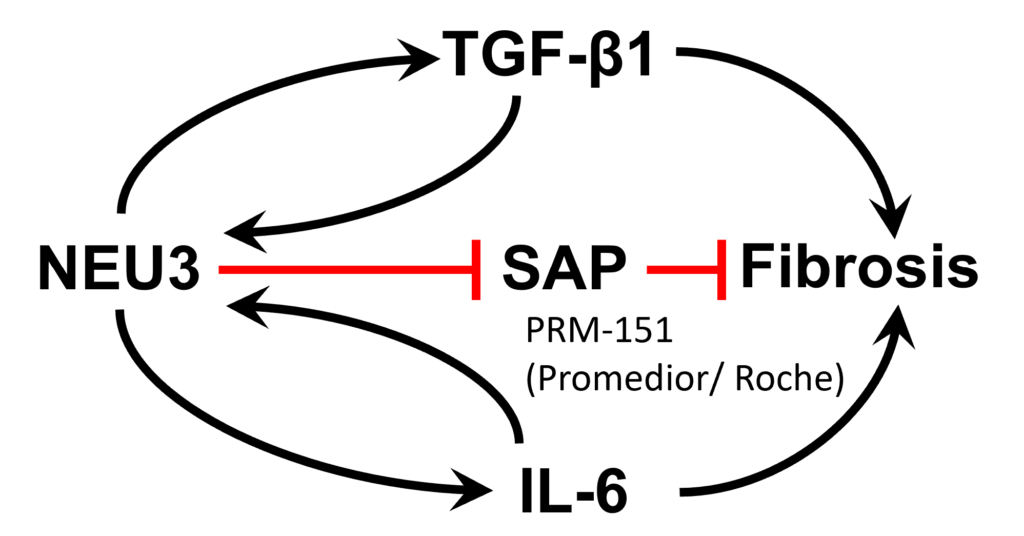
Overview of how NEU3 is a major driver of fibrosis. Our lead compounds are potent inhibitors of the NEU3 enzyme, and can block the NEU3-induced extracellular accumulation of the profibrotic signal TGF-beta1, the NEU3-induced deactivation of the antifibrotic protein SAP, and the NEU3-induced extracellular accumulation of a second profibrotic signal (IL-6). By inhibiting these effects of NEU3, our NEU3 inhibitors strongly inhibt fibrosis.
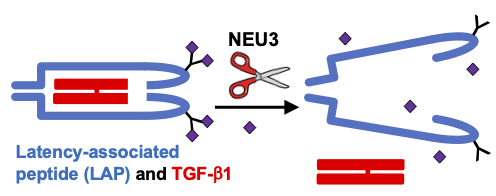
Details of how NEU3 upregulates the fibrosis driver TGFbeta1. TGFbeta1 (in red in the diagram) is wrapped and sequestered in two molecules of a protein called latency-associated peptide (LAP; blue) in the extracellular environment in tissues. LAP is a glycoprotein, and the glycosylation has one or more sialic acids (purple diamonds) at the far end of the glycosylation. NEU3 enzymatically removes the sialic acids, causing the LAP to open and release the TGF-beta1, and this can then drive fibrosis.
Target validation: Mice lacking Neu3 essentially do not develop fibrosis in the bleomycin model, and aspiration of mouse Neu3 (but not enzyme-dead Neu3) causes pulmonary fibrosis in mice. Mice lacking Neu3 are healthy; the only other observed phenotype is a resistance to colon cancer.